The industrial landscape has changed significantly in the past several years, driven by new, smarter technologies and rapidly evolving customer expectations. Smart Manufacturing, the Industrial Internet of Things (IIoT), and Artificial Intelligence (AI) are among many of the new technologies having an impact on manufacturing. Customer expectations are also changing. They want a greater range of choices and manufacturers are responding with mass customization and adaptive manufacturing – the concept of building flexibility into mass production.
The pharmaceutical industry is experiencing these changes in its own way. The number of products continues to grow, and the range of choices in mature categories is vast.
Most smart manufacturing implementations start with smart instruments. Advances in electronics have created the ability to gather more information from sensors, actuators, and analyzers. A flowmeter from 20 years ago typically supplied a flow process variable. But a modern smart flowmeter can supply hundreds of measured and calculated data points related to internal diagnostics, secondary variables such as temperature, noise signatures from the process, self-calibration functions, and other parameters (see Table 1).
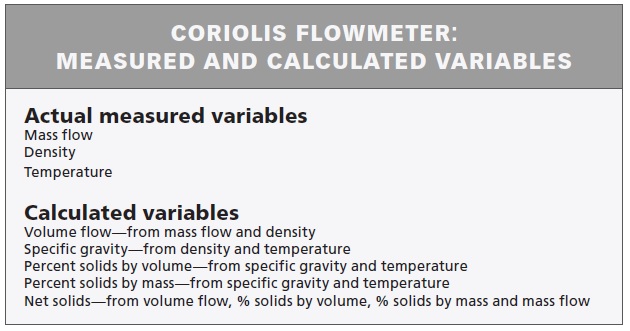
Table 1
As instruments get smarter, so do the controllers connected to them. Programmers are able to incorporate additional information from smart instruments into the process control strategy, using it to gain new insights and optimize processes.
For the pharmaceutical industry, it’s been a mixed bag. In general, pharmaceutical manufacturers use sophisticated instruments due to the high degree of precision necessary in most manufacturing processes. On the other hand, the closely regulated nature of pharmaceuticals makes it more difficult to adopt new technologies without going through the steps to have a process revalidated. Process analytical technologies (PAT) have made this process easier, but difficulties remain.
Bottom-Up or Top-Down Changes?
Most attempts to implement smart manufacturing succeed or fail based on what the user does with the data. Instruments and low-level controllers can create data. Raw materials moving around a plant can be tracked in real-time using RFID, creating data. Individuals performing manufacturing tasks have their actions recorded, creating data. But it takes higher-level systems to convert these waves of data into useful information.
Diagnostics can support highly effective maintenance programs and new instrument readings can help optimize a process, but simply packing process historians with numbers has little value. Effective enterprise-level networks and data analysis platforms are necessary to make it useful, with these implementations often referred to as industrial IT.
The automation systems used to control batch processes common to pharmaceutical manufacturing often don’t extend to the enterprise level or offer tools to help bridge the gap. Enterprise platforms must reach down to engage with these automation systems to bring the data to where it can be useful.
Pharma’s specific needs
Any consideration of pharmaceutical manufacturing must deal with the extensive regulatory environment in which it operates:
• Plants must use specific types of equipment and undergo extensive inspection
• Processes must be qualified and validated in detail
• Meticulous manufacturing records must be maintained, and
• There must be extensive traceability within the supply chain, both for raw material in and products out, often to the point of sale.
Some of these functions happen on the plant floor. Anything related to actual manufacturing processes, such as maintaining temperature in a reactor, will be controlled where it is happening, but process variables must also be maintained in batch records. Eventually the data moves up to a higher level where it is stored and used for whatever purposes are necessary.
With smart manufacturing implementations, the amount of data for a given process increases substantially. Smart instruments developed over the last few years have extensive networking capabilities, so collecting data is easier. If used properly, this data can be instructive and offer critical insights into the process.
Using a manufacturing data store

Given the amount of data collected in a manufacturing facility of any size, finding the information necessary for a given application is not always simple. Accessing the data can be made simpler by using a manufacturing data store strategy to extract the slice of information needed to fulfill a regulatory requirement, help improve a process or answer a question. A manufacturing data store is a selection and retrieval tool able to help users find and extract the information they need (e.g., Rockwell Automation’s FactoryTalk® DataMosaix™). The data store approach is used in many data-heavy applications but has not seen extensive use in pharmaceutical manufacturing.
A manufacturing data store can help gather all data related to a given lot of products, including incoming ingredients, manufacturing steps and handling, and movement within the facility. It can connect upstream to suppliers and downstream to customers, providing details on how the products were transformed from raw materials to finished pharmaceuticals.
Manufacturing data store example: product retrieval
One of the most important and compelling reasons for a manufacturing data store is handling product retrievals or recalls. Manufacturers and regulators tend to emphasize the idea of lot tracking and lot genealogy in the context of product retrievals, as if the genealogy of the materials is the only thing capable of triggering such an event.
This mindset overlooks other potential issues. The biggest part of manufacturing is the transformation of materials into finished products, which requires equipment, labor, processes (both automatic and manual), and various other activities. A more thorough evaluation requires a great deal of additional information, most of which can be accessed from a manufacturing data store.
Many different issues can trigger product retrievals, including problems with equipment, labor, processes, and so on (see Figure 1). To make an accurate determination of what exactly must be retrieved, it is necessary to have all information.
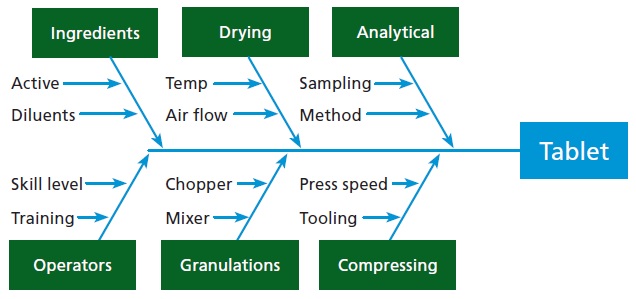
For example, if foreign objects such as metal, wood, plastic, or other trash are found in a manufacturing area, it’s necessary to know the batches that were processed in that area before the objects were discovered. The investigator needs lot tracking, but lot tracking the raw materials back doesn’t help in this situation because they are not the source of the problem. The lot tracking of everything made in the area is necessary because anything could potentially be affected back to the last time the area was inspected and known to be clean.
As another possibility, suppose that there was a problem with a piece of equipment. Maybe the agitator on a reactor wasn’t working correctly. In any case, without sufficient agitation, the reaction might not be complete, and the batch is rejected. What was going on with the agitator and reactor?
Merely having the lot genealogy doesn’t provide the information needed here. It’s necessary to get the details on that piece of equipment to determine when it might have failed. What diagnostic information might clearly define the agitator? Was the motor drawing the normal amount of current to indicate it was rotating correctly? Is some other variable, such as a temperature profile, able to suggest if the desired reaction was or was not happening? A production unit outfitted with smart instruments could easily supply that information, and a data store could provide easy access.
Continuing the examples, here is another possibility: maybe there was a problem with a particular person working in the manufacturing area. Maybe they were not certified, not trained, or they introduced a contaminant by accident. In such a case, it’s necessary to know all labor information. Again, the lot genealogy doesn’t help. What’s needed is detailed information, and it’s probably somewhere in the data. A manufacturing data store can help find it.
Traceability and more
Pharmaceutical manufacturers already practice supply chain traceability. The question to ask is how well a given company’s system works and how difficult it is to use.
Companies like UPS and FedEx do an excellent job tracking shipments, but as good as those package-tracking systems are, they don’t have sufficient capabilities to fill the larger needs of pharmaceutical manufacturers. Companies need far more detail than the location and movements of a box.
A pharmaceutical-grade product monitoring and traceability package must capture everything that goes on in a manufacturing plant, not just the movement of materials. Everything related to the manufacturing process must be recorded including incoming ingredients and raw materials, storage, manufacturing processes, personnel assignments, and equipment condition. It’s a long list of items, and no single commercially available platform can do it all.
Many pharmaceutical companies use various approaches, combining manual procedures with mixed platforms. This is where smart manufacturing often begins to break down. When there is no system capable of creating value from the data generated, the data loses its value.
Manufacturing data store concepts allow companies to build their own system customized to fit their specific needs. Implementing manufacturing data store concepts does not create more data, nor does it require reconfiguration of existing database systems. It is instead selection and retrieval tools able to help users find and extract the information they need. Many such systems have been created in a wide variety of industries, although there are few in the pharmaceutical world where they are most needed.
Smart manufacturing technologies provide many ways to automate a huge variety of manufacturing processes, and most create vast amounts of data as byproducts. A smart plant can monitor and record all its major manufacturing process elements, as well as maintenance, cleaning, quality, and safety.
Implementing a data store
Many companies have built successful data store solutions internally, which currently is the only practical option as no software providers have captured the space with comprehensive commercial product offerings. This is a mixed blessing: a solution is not a simple purchase, but simultaneously, a homegrown approach can deliver exactly the capabilities a company needs most.
Before getting bogged down in the mechanical aspects of an implementation, it is important to think through why you are doing it, and what you hope to get when it is fully realized. Pharmaceutical companies have enormous amounts of data. What information might be in there capable of supporting more effective decision-making and operational efficiencies if you could get it? Fortunately, a data store project does not have to be a huge all-at-once undertaking. It can be built-in stages beginning with the areas where it is needed most and can deliver the greatest value, hence the importance of developing the business case.
Having a partner like Rockwell Automation to help with the process can be a major advantage. The right consultant can bring project experience from other industries to help sort through the initial evaluation and implementing a program. Creating a manufacturing data store is not an easy undertaking, but as many companies have already realized, it becomes a powerful tool well worth the effort.

